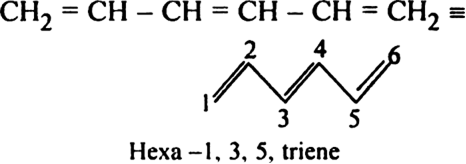Write the IUPAC names of the following compounds: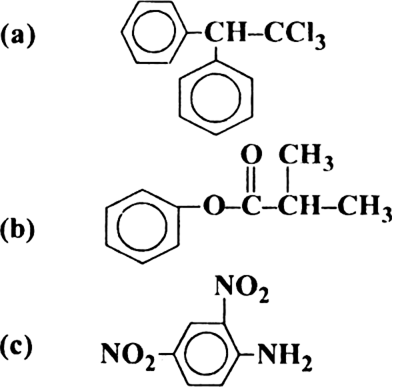
(a) 1, 1 1-Trichloro-2, 2-diphenylethane.
(b) Phenyl 2-methyl propanoate.
(c) 2, 4-Dinitrobenzenamine.
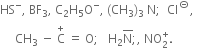
Nucleophile:

These have a unshared pair of electrons which can be donated and shared with an electrophile.
Electrophiles: 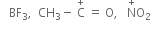
Positive sites have only six valence electrons, can accept pair from a nucleophile.
Using the curved-arrow notation, show the formation of reactive intermediate when the following covalent bonds undergo heterolytic cleavage.
(a) CH3 – SCH3 (b) CH3 – CN (C) CH3 – Cu
The curved-arrow notation of the given compound:![]()
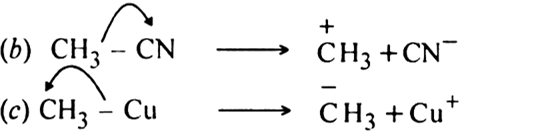
Discuss short and convenient method (bond line notations) of representing organic molecules ?
In these notations, the bonds are represented by lines and carbon atoms by line ends and intersections. For example.
(i) n-Hexane may be represented as:![]()
(ii) 2-Methylbuta-1, 3-diene may be represented as: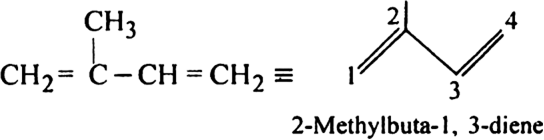
(iii) Hexa -1, 3, 5-triene may be represented as: