CBSE
Class 10 Class 12
Solids shows electrical conductivities that extend from 27 orders of magnitude ranging from 10–20 to 107 ohm–1 m–1.
Solids can be classified into three types on the basis of their conductivities.
The electrical conduction occurs when the atomic orbitals of metal atoms form molecular orbitals which are so close in energy to each other as to form a band.
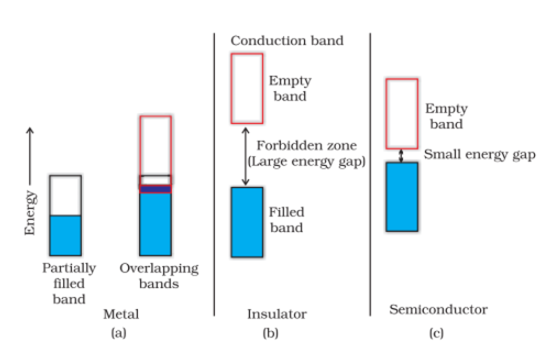
Conditions for electrical conduction:
Metal: If the band is partially filled or it overlaps with a higher energy unoccupied conduction band, then electrons can flow easily under an applied electric field and the metal shows conductivity.
Insulator: If the gap between filled valence band and the next higher unoccupied band (conduction band) is large, electrons cannot jump to it and such a substance has very small conductivity and it behaves as an insulator.
Semiconductor: In case of semiconductors, the gap between the valence band and conduction band is small. Therefore, some electrons may jump to the conduction band and show some conductivity.
A conductor may conduct electricity through movement of electrons or ions.Metals conduct electricity in solid as well as molten state. The conductivity of metals depend upon the number of valence electrons available per atom.
The electrical conductivity of a semiconductor at normal temperatures lies between a good conductor and an insulator. The range of conductivity is 10-6 to 10-4 ohm-1 m-1. Example: Silicon and germanium. Unlike metals, the conductivity of semiconductors increases with temperature because the weakly bound extra electron or positive hole become free by the increased temperature.
The conduction in the crystal without adding any external substance is called intrinsic conduction.
Doping is a process of mixing pure silicon or germanium with an impurity.
Doping enhances the conductivity and the products are called extrinsic semiconductors. They are two types:
n-type semiconductor : (Doping with electron-rich impurity)
It is obtained by doping Si or Ge with a group 15 elements i.e. as electron-rich impurities like P. Out of 5 valence electrons, only 4 are involved in the bond formation and the fifth electron is delocalized and can be easily provided to the conduction band.
p-type semiconductor: (Doping with electron deficient impurity)
It is obtained by doping Si or Ge with a group 13th elements (electron -deficient impurities) like Gallium which contains only 3 valence electrons. Due to missing of a 4th valence electron, electron-hole or electron vacancy is created. The movement of these positively charged hole is responsible for the conduction.
13 and 15 or 12 and 16 Groups compounds:
A large variety of solid materials have been prepared by a combination of groups 13 and 15 or 12 and 16 to simulate average valence of four as in Ge or Si.
Typical compounds of groups 13-15 are in Sb, AlP and GaAs. Gallium arsenide.
Typical compounds of groups 12-16 are ZnS, CdS, CdSe and HgTe.
In these compounds, the bonds are not perfectly covalent and the ionic character depends on the electronegativities of the two elements.