CBSE
Class 10 Class 12
Imperfections in Solids or Crystal defects
Irregularity in the arrangement of constituent particles in solids is called crystal defect or imperfection in solids.
There are two types of crystal defects - Point Defects and Line Defects.
Point Defects: Irregularities or deviation from the ideal arrangement of constituent particles around the point or atom in a crystalline solid is known as point defects.
Line Defects: Irregularities or deviation from the ideal arrangement of constituent particles in an entire row of the lattice is known as line defects.
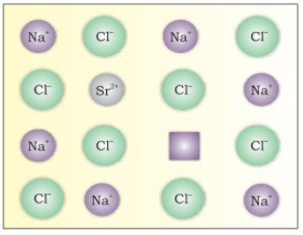
This defect occurs in ionic compounds. It arises when foreign atoms are present in lattice site.
If molten NaCl containing a little amount of SrCl2 is crystallised, some of the sites of Na+ ions are occupied by Sr2+.Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number to that of Sr2+ ions. Another similar example is the solid solution of CdCl2 and AgCl.
The defects in which stoichiometry of the compound is disturbed.
There are of two types:
I) Metal excess defects due to anionic vacancies:
II) Metal excess defects due to the presence of interstitial cations:
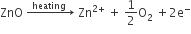
Metal deficiency due to the absence of cations: Positive ions missing from their lattice sites. Extra negative charge is balanced by some neary metal ions with higher positive charge. e.g. FeO, FeS.
A typical example of this type is FeO which is mostly found with a composition of Fe0.95O. It may actually range from Fe0.93O to Fe0.96O. In crystals of FeO some Fe2+ cations are missing and the loss of positive charge is made up by the presence of the required number of Fe3+ ions.
Point Defects: Point Defects are divided into three types:
Stoichiometric Defects: – It is a type of point defects which does not disturb the stoichiometry of solid. This is also known as Intrinsic or Thermodynamic Defects.
Types of stoichiometric defects: Vacancy Defects, Interstitial defects, Frenkel Defects, Schottky Defects.
| Vacancy Defects | Interstitial Defects |
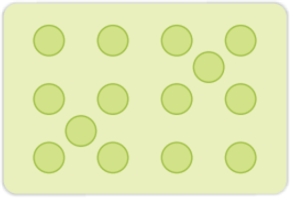
| When some lattice sites left vacant while the formation of the crystal, the defect is called Vacancy Defects. | Sometime in the formation of the lattice structure, some of the atoms occupy the interstitial site, the defect arising because of this is called Interstitial Defects. |
| In vacancy defects, an atom is missing from its regular atomic site. Because of missing of the atom the density of substance decreases, i.e. because of vacancy defects. | In interstitial defect, some atoms occupy sites at which; generally there is no atom in the crystal structure. Because of the interstitial defects, the number of atoms becomes larger than the number of lattice sites. |
| The vacancy defect develops on heating of a substance. | Increase in a number of atoms increases the density of the substance, i.e. interstitial defects increase the density of the substance. |
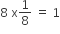 |
 |
The vacancy defects and interstitial defects are found only in non-ionic compounds. Such defects found in ionic compounds are known as Frenkel Defects and Schottky Defects.
| Schottky Defects | Frenkel Defects |
| Cation and anions in equal number are missing from the crystal lattice giving rise to vacancies or voids. | The ion, usually cation (smaller ion) instead of being in its expected location, is found in one of the interstitial/voids. |
| More common in ionic compounds with high coordination numbers.The positive and negative ions are of similar size. e.g., KBr, AgBr, NaCl, and, CsCl, KCl | There is a large difference in size between the positive and negative ions, Eg: ZnS, AgCl, AgBr and AgI |
| Since the solid with a Schottky defect contains a lesser number of ions as compared to a perfect crystal, the density of the crystal will be less as compared to that of the perfect crystal. | The density of crystals exhibiting Frenkel defect remains unchanged as the ions are present in the interstitial sites without changing the mass of the substance. |