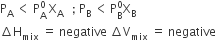CBSE
Class 10 Class 12
Liquid-liquid solutions can be classified into ideal and non-ideal solutions on the basis of Raoult’s law.
The solution which obeys Raoult's law over the entire range of concentration when enthalpy of mixing and vol. of mixing of pure component to form solution is zero.
The condition for ideal Solution
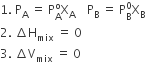
This is only possible if A-B interaction is nearly equal to those between A-A and B-B interactions. Example, a solution of n-hexane and n-heptane.
The solution which does not obey Raoult's law over the entire range of concentrations known as a Non-Ideal solution.
Conditions
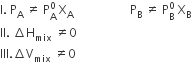
The vapour pressure of such solutions is either higher or lower than that predicted for Raoult's law.
I. If vapour pressure is higher, the solutions show positive deviation (A-B interaction are weaker than those between A-A and B-B).
Example: Mixture of ethanol and acetone.
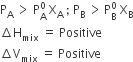
II. If vapour pressure is lower, the solution shows negative deviation (A-B interaction are stronger than those between A-A and B-B).