CBSE
Class 10 Class 12
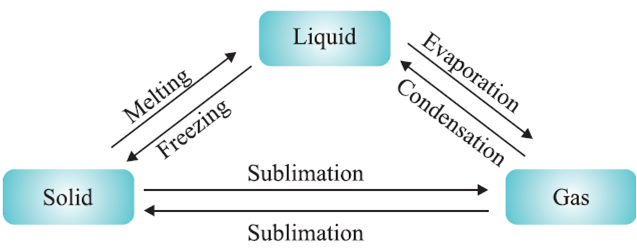
Change of State of Matter :
The process of change of states of matter:
Change in the physical state of matter can be done in two ways :
If we compress a gas in a cylinder, the distance between the particles of gas is reduced and finally, gas is liquefied on lowering temperature.

On increasing the temperature of solids, the kinetic energy of the particles increases. Due to the increase in kinetic energy, the particles start vibrating with greater speed.
The energy supplied by heat overcomes the forces of attraction between the particles. The particles leave their fixed positions and start moving more freely. A stage is reached when the solid melts and is converted to a liquid.
Melting Point: The temperature at which a solid melts to form a liquid at atmospheric pressure is called its melting point. The melting point of ice is 273.16 K (0º C).
During melting the temperature of ice does not rise even though heat is being supplied continuously due to the latent heat of fusion.
Latent Heat of Fusion: The amount of heat required to change 1 kg solid to its liquid state (at its melting point) at atmospheric pressure.
Boiling Point: The temperature at which a liquid boils to form vapours at atmospheric pressure is called its boiling point. Boiling point of water is 373 K (100º C + 273 = 373 K).
Latent Heat of Vapourization: The amount of heat required to change 1 kg liquid to its gaseous state (at its boiling point) at atmospheric pressure.
During boiling the temperature of the water does not rise even though heat is being supplied continuously as this heat of vaporization is used up to over the forces of attraction between water particles.
At 100º C, the energy of water vapours is much more than the energy of water at 100º C. So, we can change one state of matter to another state by changing temperature.

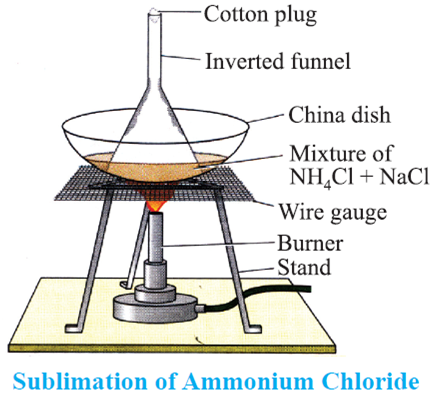
Sublimation: The change of solid directly into vapours on heating and of vapours into solid on cooling without passing through the intervening liquid state is called sublimation.
Example: When camphor or ammonium chloride is heated in a China a dish covered by an inverted funnel (with a cotton plug in its upper open end), the vapours of ammonium chloride are converted into solid ammonium chloride on coming in contact with the cold inner walls of the funnel.