CBSE
Class 10 Class 12
Neils Bohr, a Danish physicist, in 1913 proposed model of the atom which rectified the problems left by Rutherford’s Model. He proposed that
Radiate energy as long as they keep on revolving around the nucleus in a fixed orbit.
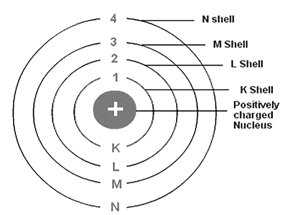
The circular path around the nucleus is called orbit, energy level or shell. Energy level is represented by letter – K, L, M, N, .... and so on.
Therefore,
1st orbit is denoted by – K
2nd orbit is denoted by – L
3rd orbit is denoted by – M, and so on.
The orbits are denoted by 1, 2, 3, .... and so on.
Neils Bohr, a Danish physicist, in 1913 proposed model of the atom which rectified the problems left by Rutherford’s Model. He proposed that
Radiate energy as long as they keep on revolving around the nucleus in a fixed orbit.
The circular path around the nucleus is called orbit, energy level or shell. Energy level is represented by letter – K, L, M, N, .... and so on.
Therefore,
1st orbit is denoted by – K
2nd orbit is denoted by – L
3rd orbit is denoted by – M, and so on.
The orbits are denoted by 1, 2, 3, .... and so on.

Rutherford was interested in knowing how the electrons are arranged within an atom.
Rutherford designed an experiment in this fast moving alpha (α)-particles were made to fall on a thin gold foil.
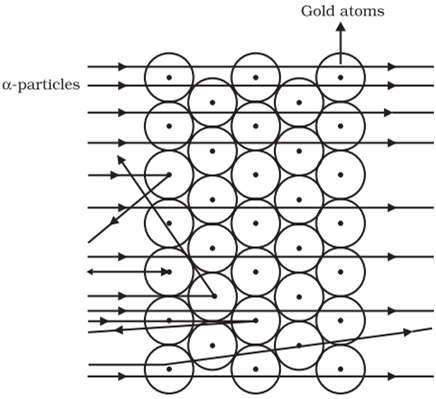
The following observations were made:
Rutherford concluded from the α-particle scattering experiment that:
He also calculated that the radius of the nucleus is about 105 times less than the radius of the atom.
Rutherford put forward the nuclear model of an atom:
Drawbacks of Rutherford’s model of the atom:
J.J Thomson proposed the model of atom similar to a Christmas pudding or similar to a watermelon. His model of the atom is generally called plum and pudding model of the atom.
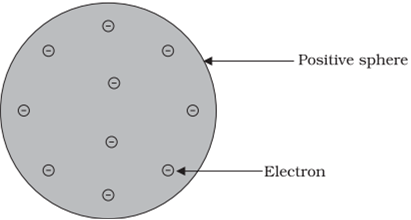
He proposed that electron is embedded the way black seeds of watermelon are embedded; In the sphere of positive charge.
According to Thomson-
a) An atom consists of the positively charged sphere in which electrons are embedded.
b) The quanta of negative and positive charges are equal. Thus, the atom is electrically neutral.