CBSE
Class 10 Class 12
Atomic models were proposed to explain the distributions of these charged particles in an atom.
The charge on the proton is equal but opposite to that of the electron. The number of protons present in the nucleus is equal to the atomic number (Z )
For example, the number of protons in the hydrogen nucleus is 1, in sodium atom it is
11, therefore their atomic numbers are 1 and 11 respectively.
Atomic number (Z) = Number of protons in the nucleus of an atom or it can be defined number of electrons present in a neutral atom.
Mass number (A): Atomic mass can be defined as, the sum of a number of protons (Z) and a number of neutrons (n).
Isobars are the atoms with the same mass number but a different atomic number.
For example,.
Isotopes are the atoms with an identical atomic number but a different atomic mass number is known as Isotopes.
For example,
The general representation of the atomic number and atomic mass can be given as,
Element symbol (X) with super-script on the left-hand side as the atomic mass number (A) and subscript (Z) on the left-hand side as the atomic number.
Rutherford was interested in knowing how the electrons are arranged within an atom.
Rutherford designed an experiment in this fast moving alpha (α)-particles were made to fall on a thin gold foil.
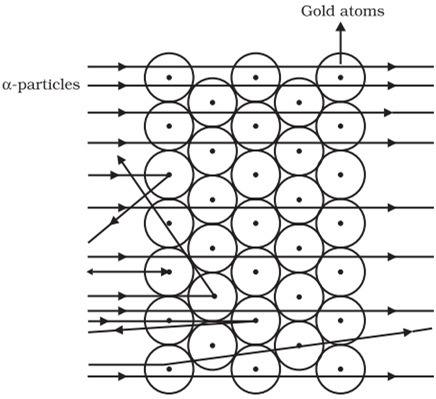
The following observations were made:
Rutherford concluded from the α-particle scattering experiment that:
He also calculated that the radius of the nucleus is about 105 times less than the radius of the atom.
Rutherford put forward the nuclear model of an atom:
Drawbacks of Rutherford’s model of the atom:
J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed.
It is also known as Thomson’s plum pudding model.
Thomson model of atom was discarded because it could not explain the overall neutrality of the atom.