 Multiple Choice Questions
Multiple Choice QuestionsThe equilibrium constant at 298 K for a reaction A+B ⇌ C+D is 100. If the initial concentration of all the four species were 1 M each, then equilibrium concentration of D (in mol L−1 ) will be:
0.818
1.818
1.182
1.182
For the reaction,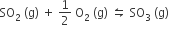
if Kp = Kc (RT)x where the symbol has usual meaning then the value of x is (assuming ideality)
-1
-1/2
1/2
1/2
The species which can best serve as an initiator for the cationic polymerization is
LiAlH4
HNO3
AlCl3
AlCl3
The equilibrium constant (Kc) for the reaction, N2(g) + O2 (g) → 2NO (g) at temperature T is 4 x 10-4. The value of Kc for the reaction 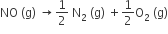 at the same temperature is
at the same temperature is
0.02
2.5 x 102
4 x 10-4
4 x 10-4
The pH of a 0.1 molar solution of the acid HQ is 3. The value of the ionisation constant, Ka of this acid is
3 x 10–1
1 x 10–3
1 x 10—5
1 x 10—5
Consider the reaction:
Cl2(aq) + H2S(aq) → S (s) + 2H+ (aq) + 2Cl- (aq)
The rate equation for this reaction is
I. Cl2 + H2S → H+ +Cl- + Cl+ +HS-
II. H2S ⇌ H+ + HS- (fast equilibrium)
Cl2 + HS- → 2Cl- + H+ + S (slow)
II only
Both (I) and (II)
Neither (I) nor (II)
Neither (I) nor (II)
Three reactions involving H2PO4- are given below
I. H3PO4 + H2O → H3O+ + H2PO4-
II. H2PO4- + H2O→ HPO42- + H3O+
III. H2PO4- + OH- → H3PO4 + O2-
In which of the above does H2PO4- act as an acid
(II) only
(I) and (II)
(III) only
(III) only
In aqueous solution the ionization constants for carbonic acid are
K1 = 4.2 x 10-7 and K2 = 4.8 x 10-11.
Select the correct statement for a saturated 0.034 M solution of the carbonic acid.
The concentration of CO32- is 0.034 M
The concentration of CO32- is greater than that of HCO3-
The concentration of H+ and HCO3- are approximately equal
The concentration of H+ and HCO3- are approximately equal
At 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?
9
10
11
11
