 Multiple Choice Questions
Multiple Choice QuestionsThe species in which the N atom is in a state of sp hybridization is:
NO2-
NO3-
NO2
NO2
The ionic radii (in Å) of N3–, O2– and F– are respectively:
1.36, 1.40 and 1.71
1.36, 1.71 and 1.40
1.71, 1.40 and 1.36
1.71, 1.40 and 1.36
In which of the following pairs of molecules/ions, both the species are not likely exist?


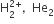
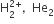
Which of the following exists as covalent crystals in the solid state?
Iodine
Silicon
Sulphur
Sulphur
Stability of the species Li2, Li2− and Li2+ increases in the order of:
Li2 < Li2+ < Li2-
Li2− < Li2+ < Li2
Li2< Li2− < Li2+
Li2< Li2− < Li2+
In which of the following pairs the two species are not isostructural?
CO32- and NO3-
PCl+4 and SiCl4
PF5 and BrF5
PF5 and BrF5
Among the following, the maximum covalent character is shown by the compound
FeCl2
SnCl2
AlCl3
AlCl3
The hybridization of orbitals of N atom in NO3-, NO2+ and NH4+ are respectively
sp, sp2,sp3
sp2 , sp, sp
sp, sp3 , sp2
sp, sp3 , sp2
The group having isoelectronic species is
O2– , F–, Na+ , Mg2+
O– , F–, Na, Mg+
O2–, F–, Na ,Mg2+
O2–, F–, Na ,Mg2+
Using MO theory predict which of the following species has the shortest bond length?
O22+
O2+
O2−
O2−
