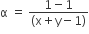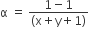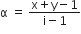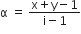Multiple Choice Questions
Multiple Choice QuestionsWhich among the following metals is employed to provide cathodic protection to iron?
Zinc
Nickel
Tin
Lead
Two Faraday of electricity is passed through a solution of CuSO4. The mass of copper deposited at the cathode is: (at. mass of Cu = 63.5 amu)
0 g
63.5 g
2 g
2 g
The equivalent conductance of NaCl at concentration C at infinite dilution are λC and λ∞ respectively. The correct relationship between λC and λ∞ is given as (where the constant B is positive)
λC = λ∞ +(B)C
λC = λ∞ -(B)C
λC = λ∞ -(B)
λC = λ∞ -(B)
Given below are the half-cell reactions
Mn2+ + 2e- → Mn; Eo = - 1.18 eV
2(Mn3+ + e- →Mn2+); Eo = +1.51 eV
The Eo for 3Mn2+ → Mn + 2Mn3+ will be
-2.69 V; the reaction will not occur
-2.69 V; the reaction will occure
-0.33 V; the reaction will not occur
-0.33 V; the reaction will not occur
Given, 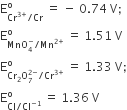
Based on the data given above, strongest oxidising agent will be
Cl
Cr3+
Mn2+
Mn2+
Four successive members of the first-row transition elements are listed below with atomic numbers. Which one of them is expected to have the highest 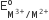 value?
value?
Cr (Z =24)
Mn(Z =25)
Fe (Z = 26)
Fe (Z = 26)
How many litres of water must be added to 1 L to an aqueous solution of HCl with a pH of 1 create an aqueous solution with PH of 2?
0.1 L
0.9 L
2.0 L
2.0 L
The reduction potential of hydrogen half-cell will be negative if
p(H2) = 1 atm and [H+] = 2.0 M
p(H2) = 1 atm and [H+] = 1.0 M
p(H2) = 2 atm and [H+] = 1.0 M
p(H2) = 2 atm and [H+] = 1.0 M
The degree of dissociation (α) of a weak electrolyte ,AxBy is related to van't Hoff factor (i) by the expression