 Short Answer Type
Short Answer TypeShow how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal?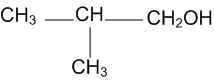

 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type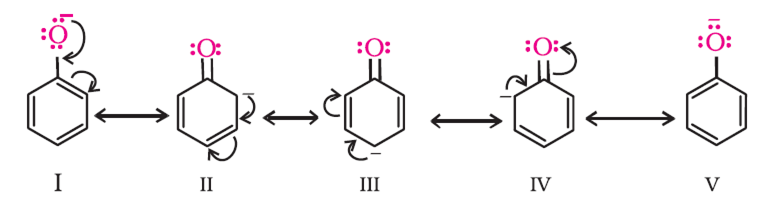
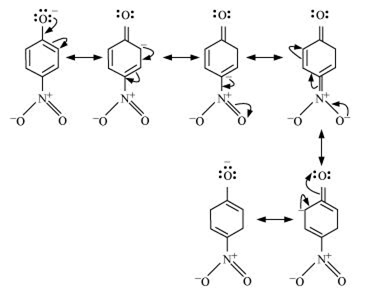
Resonance structures of ortho nitro phenol: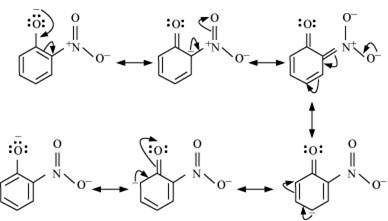
Thus, presence of nitro group at ortho and para position increase the acidic character.
