 Short Answer Type
Short Answer TypeName the ether used as inhalation an aesthetic. What are the disadvantages of using it?
How would you differentiate between phenol and carboxylic acid by a simple chemcial test?
Alcohols can form H- bonds with due to presence of (-OH) group and break the H-bonds already existing between water molecules. Hence they are soluble in water.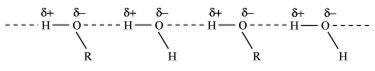
In contrast, hydrocarbon cannot form H-bonds with water and hence are insoluble in water.
Of the two hydroxy organic compounds ROH and R'OH, the first one is basic and the other is acidic in behaviour. How is R different from R'?
Phenol is acidic but does not decompose NaHCO3 solution while carbonic acids like CH3COOH decompose NaHCO3 solution. Why?
