 Short Answer Type
Short Answer TypeIf an alcohol is treated with excess of sulphuric acid, no esterification takes place. Why?
How many sigma bonds are present in 3-methyl phenol?
Structure of 3-methyl phenol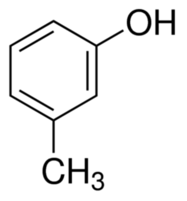
6 (benzene ring) + 4(C—H of benzene ring) +1 (C of benzene ring and CH3 group) +3 (of CH3 group) + 1(C of benzene and O of OH) +1 (O and H of OH group) = 16.
Ethanol and chloro ethane both are polar in nature yet ethanol is miscible in water while chloro ethane is immiscible. Explain.
