 Short Answer Type
Short Answer TypeOrtho nitrophenol is more acidic than ortho-methoxyphenol. This is because the nitro-group is an electron-withdrawing group. The presence of this group in the ortho position in ortho nitro phenol decreases the electron density in the O−H bond. As a result, it is easier to lose a proton.Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid.
In the case of ortho methoxyphenol; methoxy group is an electron-releasing group. Thus, it increases the electron density in the O−H bond and hence, the proton cannot be given out easily. Hence, ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Arrrange the following groups of compounds in order of decreasing acidity: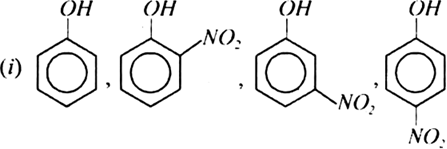
(ii) Phenol, o-chlorophenol, m-chlorophenol, p-chlorophenol.
(iii) o-cresol, m-cresol, p-cresol, phenol.
 Long Answer Type
Long Answer TypeWrite the structures of the major products expected from the following reactions:
(a) Mononitration of 3-methylphenol.
(b) Dinitration of 3-methylphenol.
(c) Mononitration of phenyl methanoate.
 Short Answer Type
Short Answer TypeHow are the following conversions carried out?
Ethyl magnesium chloride → Propane- 1-ol.
