 Short Answer Type
Short Answer TypeUse simple chemical test to differentiate between the following pairs of organic compounds:
(i) 2, 4, 6-trinitro phenol and 2, 4, 6-tri methyl phenol.
(ii) Anisole and benzyl alcohol.
Give a chemical test to distinguish between: 2-pentanol and 3-pentanol.
This compound has a CH3C = O group.
Therefore, it will form iodoform (CHI3) with iodine and alkali on warming. Iodoform is a yellow crystalline substance.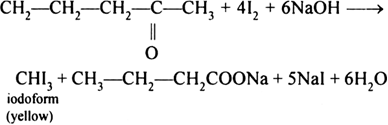
3-pentanol does not show this test.
Write the resonance structures of phenol (which is similar to that of chlorobenzene) and explain why it does not form chlorobenzene on reaction with PCI5 Explain whether phenol can be dehydrated on heating with catalytic amounts of conc. H2SO4.
Account for the following:
Alcohols with three or less carbons are water-soluble while alcohols with five or more carbons are insoluble.
Write the reaction for the preparation of the following:
n-propyl chloride from n-propyl alcohol.
Write the reaction for the preparation of the following:
2, 4-dinitro phenol from 2, 4-dinitro chlorobenzene;
