 Short Answer Type
Short Answer TypeAlthough phenoxide ion has more number of resonating structures than Carboxylate ion, Carboxylic acid is a stronger acid than phenol. Give two reasons.
 Long Answer Type
Long Answer TypeGive simple chemical tests to distinguish between the following pairs of compounds:
(i) Ethanal and Propanal
(ii) Benzoic acid and Phenol
(i) Distinguish test between ethanal and propanal:
Iodoform Test: Ethanal gives iodoform test.
CH3CHO + 4NaOH + 3I2 ---> CHI3 (Yellow ppt.) + HCOONa + 3NaI + 3H2O
Propanal does not give this test.
CH3CH2CHO + 4NaOH + 3I2 ---> No Reaction.
(ii) Distinguish test between Benzoic acid and Phenol:
NaHCO3Test: When Benzoic acid reacts with NaHCO3, the brisk effervescence of CO2 gas evolved.
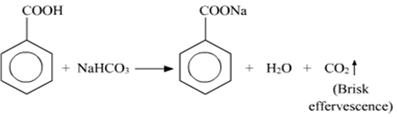
Phenol does not give this test.
C6H5OH + NaHCO3 --> No Reaction
 Short Answer Type
Short Answer TypeArrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions: ethanol, propanal, propanone, butanone.
Draw the structure and name the product formed if the following alcohols are oxidised. Assume that an excess of oxidising agent is used.
(i) CH3CH2CH2CH2OH
(ii) 2-butenol
(iii) 2-methyl-1-proponal
Write chemical equation for the following conversions:
(i) Nitrobenzene to benzoic acid.
(ii) Benzyl chloride to 2-phenylethanamine.
(iii) Aniline to benzyl alcohol.
How would you obtain
(i) Picric acid (2, 4, 6-trinitrophenol) from phenol,
(ii) 2-Methylpropene from 2-methylpropanol?
How do you convert the following:
(i) C6H5CONH2 to C6H5NH2
(ii) Aniline to phenol
(iii) Ethanenitrile to ethanamine
