 Short Answer Type
Short Answer TypeWrite the chemical equations involved when aniline is treated with the following reagents:
(i) Br2 water
(ii) CHCI3 + KOH
(iii) HCl
Explain the following behaviours:
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitro phenol is more acidic than ortho-methoxyphenol.
Explain the mechanism of acid catalysed hydration of an alkene to form corresponding alcohol.
Some reactive alkenes undergo direct hydration in the presence of mineral acids which act as catalysts. The addition of water to the double bond takes place in accordance with Markonikoff’s rule.
The mechanism of reaction involves the following three steps:
(i) Protonation of alkene to form carbocation by electrophilic attack of H3O+.
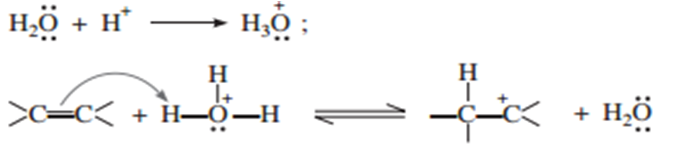
(ii) Nucleophilic attack of water of carbocation.
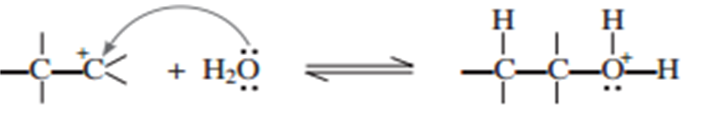
(iii) Deprotonation to from an alcohol.

Write the equations involved in the following reactions:
(i) Reimer − Tiemann reaction
(ii) Williamson synthesis
