 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of acid strength of the following substituted phenols in water at 28°C is
p-nitrophenol < p-fluorophenol < p-chlorophenol
p-chlorophenol < p-fluorophenol < p-nitrophenol
p-fluorophenol < p-chlorophenol < p-nitrophenol
p-flurophenol < p-nitrophenol < p-chlorophenol
Correct statement(s) in cases of n-butanol and t-butanol is (are)
both are having equal solubility in water
t-butanol is more soluble in water than n-butanol
boiling point of t-butanol is lower than n-butanol
boiling point of n-butanol is lower than t-butanol
Two aromatic compounds having formula C7H8O which are easily identifiable by FeCl3 solution test (violet colouration) are
o-cresol and benzyl alcohol
m-cresol and p-cresol
o-cresol and p-cresol
methyl phenyl ether and benzyl alcohol
Which one of the following methods is used to prepare Me3COEt with a good yield?
Mixing EtONa with Me3CCl
Mixing Me3CONa with EtCl
Heating a mixture of (1:1) EtOH and Me3COH in the presence of conc. H2SO4
Treatment of Me3COH with EtMgI
The ease of dehydrohalogenation of alkyl halide with alcoholic KOH is
3° < 2° < 1°
3° > 2° > 1°
3° < 2° > 1°
3° > 2° < 1°
The correct order of decreasing acidity of nitrophenols will be
m-nitrophenol > p-nitrophenol > o-nitrophenol
o-nitrophenol > m-nitrophenol > p-nitrophenol
p-nitrophenol > m-nitrophenol > o-nitrophenol
p-nitrophenol > o-nitrophenol > m-nitrophenol
D.
p-nitrophenol > o-nitrophenol > m-nitrophenol
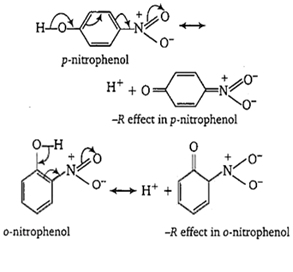
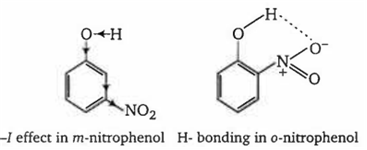
-NO2 group at o- and p- position withdraws electrons of the O-H bond towards itself by the stronger -R effect while the NO2 group at m-position with draws electrons of the O-H bond by the weaker -I effect. Thus, o- and p- nitrophenols are more acidic than m- nitrophenol.
Among o- and p-nitrophenols, o- nitrophenol is little less acidic than p-nitrophenol due to intramolecular H-bonding which makes loss of a proton little more difficult.
Which one of the following properties is exhibited by phenol?
It is soluble in aq. NaOH and evolves CO2 with aq. NaHCO3,
It is soluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
It is not soluble in aq. NaOH but evolves CO2 with aq. NaHCO3
It is insoluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
