 Multiple Choice Questions
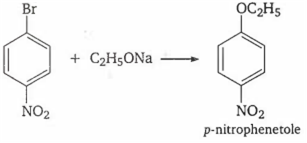
Multiple Choice QuestionsWhen p-nitrobromobenzene reacts with sodium ethoxide, the product obtained is
p-nitroanisole
ethyl phenyl ether
p-nitrophenetole
no reaction occurs
C.
p-nitrophenetole
In general aryl halides are highly stable and do not take part in Williamson's synthesis, but presence of strong electron withdrawing group like NO2 makes the C-X bond weaker and facilitate the substitution of -Br by -OR.
Hydroboration oxidation of 4-methyl octene would give
4-methyl octanol
2-methyl decane
4-methyl heptanol
4-methyl-2-octanone
When ethyl alcohol is heated with conc. H2SO4, the product obtained is
CH3COOC2H5
C2H2
C2H6
C2H4
Anisole is the product obtained from phenol by the reaction known as
coupling
etherification
oxidation
esterification
The epoxide ring consists of which of the following
three membered ring with two carbon and one oxygen
four membered ring with three carbon and one oxygen
five membered ring with four carbon and one oxygen
six membered ring with five carbon and one oxygen
In the Grignard reaction, which metal forms an organometallic bond?
Sodium
Titanium
Magnesium
Palladium
Trans esterification is the process of :
conversion of an aliphatic acid to ester
conversion of an aromatic acid to ester
conversion of one ester to another ester
conversion of an ester into its components namely acid and alcohol
Phenols are more acidic than alcohols because
phenoxide ion is stabilised by resonance
phenols are more soluble in polar solvents
phenoxides ions do not exhibit resonance
alcohols do not lose H atoms at all
