 Multiple Choice Questions
Multiple Choice QuestionsEther and alcohol are ...... isomers.
chain
position
functional
not isomers
C.
functional
General formula for both ethers and alcohols is CnH2n+2 but the functional group in ethers is -O- and in alcohols is -OH. Therefore, they are functional isomers.
In the following reaction,
the product is
ethene
ethyl hydrogen sulphate
diethyl ether
acetylene
Phenol gives characteristic colouration with
iodine solution
bromine water
aqueous FeCl3 solution
ammonium hydroxide
In the following sequence of reactions
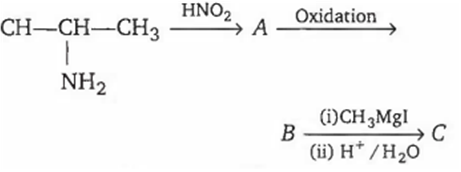
The compound C formed will be
butanol-1
butanol-2
2-methyl propanol-1
1, 1-dimethylethanol
The correct order of dehydration of alcohols is
1° > 2° > 3°
3° > 2° > 1°
2° > 1° > 3°
1° > 3° > 2°
Ethanolic KOH gives
dehalogenation reactions
dehydrogenation reactions
dehydrohalogenation reactions
substitution reactions
Which alcohol will give immediate turbidity on shaking with HCl at room temperature?
3-methyl pentan-2-ol
2-methyl butan-1-ol
Butan-3-ol
2-methylpropan-2-ol
