 Multiple Choice Questions
Multiple Choice QuestionsThe compound of molecular formula C5H10O(A) reacts with Tollen's reagent to give silver mirror but does not undergo aldol condensation. The compound A is
3-pentanone
2, 2-dimethylpropanal
3-hydroxy-2-pentene
3-methylbutanal
lsopropyl methyl ether when treated with cold hydrogen iodide gives
isopropyl iodide and methyl iodide
isopropyl alcohol and methyl iodide
isopropyl alcohol and methyl alcohol
isopropyl iodide and methyl alcohol
Compound 'A' of molecular formula C4H10O on treatment with Lucas reagent at room temperature gives compound 'B'. When compound 'B' is heated with alcoholic KOH, it gives isobutene. Compound 'A' and 'B' are respectively
2-methyl-2-propanol and 2-methyl-2-chloropropane
2-methyl-1-propanol and 1-chloro-2-methylpropane
2-methyl- 1-propanol and 2-methyl-2-chloropropane
butan-2-ol and 2-chlorobutane
Salicylaldehyde can be prepared from phenol by
Scholten-Baumann reaction
Kolbe's reaction
Reimer-Tiemann reaction
Perkin reaction
Which among the following phenolic compound is most acidic in nature?
p-aminophenol
Phenol
m-nitrophenol
p-nitrophenol
Isopropylbenzene is oxidized in the presence of air to compound 'A'. When compound 'A' is treated with dilute mineral acid, the aromatic product formed is
phenol
benzene
benzaldehyde
acetophenone
A.
phenol
The aromatic product formed is phenol. It is the commercial method for the manufacture of phenol. The reaction is as follows-
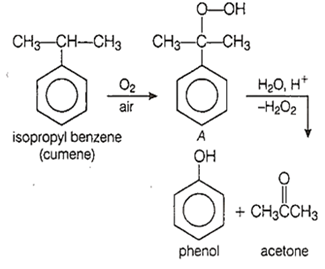
Name the catalyst used in commercial method of preparation of phenol.
Silica
Calcium phosphate
Anhydrous aluminium chloride
Cobalt naphthenate
The molecular formula of Wilkinson's catalyst used in the hydrogenation of alkenes is
Co(CO)8
(Ph3P)3RhCl
[Pt(NH3)2Cl2]
K[Ag(CN)2]
Phenol can be converted to o-hydroxybenzaldehyde by
Kolbe's reaction
Reimer-Tiemann reaction
Wurtz reaction
Cannizaro reaction
When 3-phenylpropene reacts with HBr in the presence of peroxide, the major product formed is
2-bromo 1-phenylpropane
1, 2-dibromo 3-phenylpropane
3-(o-bromophenyl) propene
1-bromo 3-phenylpropane
