 Multiple Choice Questions
Multiple Choice QuestionsIdentify A and B in the following reaction
A - HI + red P; B - LiAlH4
A - Ni/ ; B - LiAlH4
A - LiAlH4 ; B - HI + red P
A - Pd- BaSO4 ; B - Zn - HCl
| Reactants | Product |
| (A) C2H5Cl, most Ag2O | (i) CH3CH2ONO |
| (B) C2H5Cl, aqueous ethanolic AgCN | (ii) C2H4 |
| (C) C2H5Cl, aqueous ethanolic AgNO2 | (iii) CH3CH2OH |
| (D) C2H5Cl, ethanolic KOH | (iv) CH3CH2NC |
| (v) C2H6 |
A-v, B-iii, C-iv, D-i
A-i, B-ii, C-iii, D-iv
A-iii, B-iv, C-i, D-ii
A-iv, B-i, C-ii, D-v
The IUPAC name of the compound (CH3)2CH—CH=CHCHOH—CH3 is
5-methyl-hex-3-en-2-ol
2-methyl-hex-3-en-5-ol
2-hydroxy-5-methyl-3-hexene
5-hydroxy-2-methyl-3-hexene
The conversion of O-acylated phenol in presence of AlCl3 to C-acylated phenol is an example for this type of organic reaction
addition reaction
substitution reaction
molecular rearrangement
elimination reaction
Consider the following reaction-
C2H5Cl + AgCN X (major)
Which one of the following statements is true for X ?
(I) It gives propionic acid on hydrolysis.
(II) It has an ester functional group.
(III) It has a nitrogen linked to ethyl carbon.
(IV) It has a cyanide group.
IV
III
II
I
The order of reactivity of phenol (I), nitrobenzene (II) and benzene (III) towards nitration is
(III) > (I) > (II)
(II) > (III) > (I)
(I) > (III) > (II)
(I) > (II) > (III)
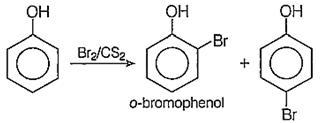
The products formed, in the reaction of phenol with Br2 dissolved in CS2 at 0°C are
o-bromo, m-bromo and p-bromophenols
o-bromo and p-bromophenols
2,4,6-tribromo and 2,3,6-tribromophenols
2,4-dibromo and 2,6-dibromophenols
B.
o-bromo and p-bromophenols
Phenol when treated with Br2 in the presence of non-polar solvent CS2, it gives only o- and p-bromophenols instead of the tri-substituted products. This is because the supression of phenoxide ion in non-polar solvent. Thus, we get only mono-substituted products.
C2H5OH + 4I2 + 3Na2CO3 → X + HCOONa + 5NaI + 3CO2 + 2H2O
In the above reaction, 'X' is
diodomethane
triodomethane
iodomethane
tetrariodomethane
