 Multiple Choice Questions
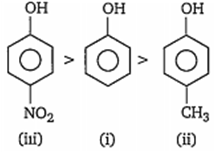
Multiple Choice QuestionsCorrect acidic order of the following compounds is

i > ii> iii
iii> i> ii
ii> iii> i
i> iii> ii
B.
iii> i> ii
Presence of electron withdrawing group such as NO2 CHO etc, on benzene nucleus, makes phenol more acidic by stabilising phenoxde ion while presence of electron releasing groups such as -CH3, -C2H5, destabilises the phenoxide ion, thus makes the phenol less acidic. Hence, the order of acidity of given compounds is
Identify Z in the following series
C2H5OH
CH2 = CH2
CH3-CH2OH
CH3-CH2-O-CH2-CH3
None of the above
The following compounds have identical molecular weight. Which would have the lowest boiling point?
2-butanol
2-methyl-1-propanol
1, 1-dimethyl ethanol
1-methoxypropane
CO2 acts as electrophile in which reaction?
Williamson's reaction
Kolbe reaction
Perkin's reaction
Reimer-Tiemann reaction
Except one, the other three are isomers, find odd man out
| ethanol | oxiran | oxitane | vinyl alcohol |
| (1) | (2) | (3) | (4) |
vinyl alcohol
ethanol
oxiran
oxitane
Glycerol, ethanol, glycol and methanol have same price per kilogram. Which liquid will be cheaper in preparing antifreeze,solution for automobile radiators?
Methanol
Ethanol
Glycol
Glycerol
Which of the following combinations can be used to synthesise ethanol?
CH3MgI and CH3COCH3
CH3MgI and C2H5OH
CH3MgI and CHCH3COOC2H5
CH3MgI and HCOOC2H5
Cumene process is the most important commercial method for the manufacture of phenol. Cumene is
1-methyl ethyl benzene
ethyl benzene
vinyl benzene
propyl benzene
A gas formed by the action of alcoholic KOH on ethyl iodide, decolourises alkaline KMnO4. The gas is
C2H6
CH4
C2H2
C2H4
