 Multiple Choice Questions
Multiple Choice QuestionsThe function of AlCl3 in Friedel-Craft's reaction is
to absorb HCl
to absorb water
to produce nucleophile
to produce electrophile
D.
to produce electrophile
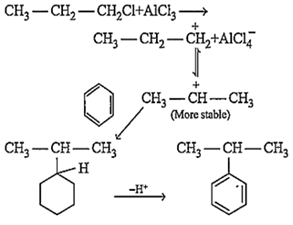
The function of AlCl3, in Friedel Craft's reaction, is to produce electrophile, which later add to benzene nucleus
Phenol
Name of the above reaction is
Liebermann's reaction
Phthalein fusion test
Reimer-iemann reaction
Schotten-Baumann reaction
The silver salt of a fatty acid on refluxing with an alkyl alide gives an
acid
ester
ether
amine
A compound A has a molecular formula C2Cl3OH. It reduces Fehling's solution and on oxidation, gives a monocarboxylic acid B. A can be obtained by the action of chlorine on ethyl alcohol. A is :
chloroform
chloral
methyl chloride
monochloroacetic acid
The reaction in which phenol differs from alcohol is:
it undergoes esterification with carboxylic acid
it reacts with ammonia
it forms yellow crystals of iodoform
it liberates H2 with Na metal
An organic compound A containing C, H and O has a pleasant odour with boiling point of 78°C. On boiling A with concentrated H2SO4, a colourless gas is produced which decolourises bromine water and alkaline KMnO4. The organic liquid A is:
C2H5Cl
C2H5COOCH3
C2H5OH
C2H6
The catalyst used in the preparation of an alkyl chloride by the action of dry HCl on an alcohol is:
anhydrous AlCl3
FeCl3
anyhydrous ZnCl2
Cu
Which of the following compound would not evolve CO2 when treated with NaHCO3 solution?
Salicylic acid
Phenol
Benzoic acid
4-nitrobenzoic acid
