 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following represents the correct decreasing order of acidity of the following compounds?
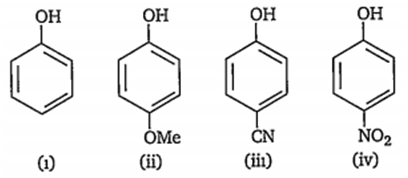
ii > i > iii > iv
iv > iii > ii > i
iii > iv > i > ii
iv > iii > i > ii
How much ethyl alcohol C2H5OH, must be added to 1.00 L of water so that the solution will not freeze at - 4°F?
211 gm
495 gm
85 gm
46 gm
What will be the major product when 2-amino propane is treated with nitrous acid?
Propane-2-ol
Cyclopropene
Propanol
2-rntropropane
Which of the following compounds is most acidic?
![]()
![]()
![]()
Cl-CH2-CH2-OH
A.
![]()
The correct order of acidic strength is
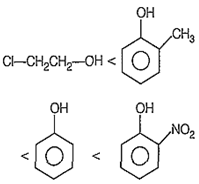
Phenols are stronger acids than alcohols because the phenoxide ion left after the release of a proton is stabilised by resonance but the alkoxide ion is not. Further, the electron-withdrawing groups (-NO2) whch stabilise the phenoxide, on by dispersing the negative charge relative to phenol increase the acidic strength of phenols, whereas electron donating group (-CH3) which destabilise the phenoxide ion by intensifying the negative charge relative to phenol tend to decrease the acidity of phenols.
Phenol + CCl4 + KOH → X;
Which of the following statement is true for X?
It gives effervescence with NaHCO3
Gives silver mirror with Tollen's reagent
Does not give the red colour with FeCl3
All of the above
Which of the following compounds contain atleast one secondary alcohol?
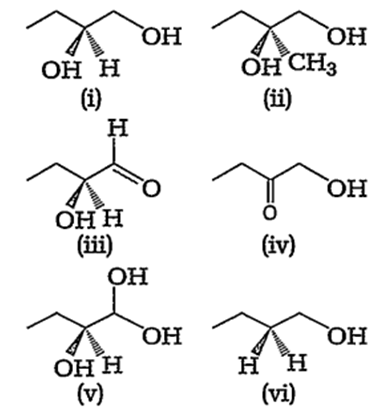
(i), (ii), (iii), (v)
(i), (iii), (v)
(i), (ii), (iv), (vi)
(i), (ii), (iii)
