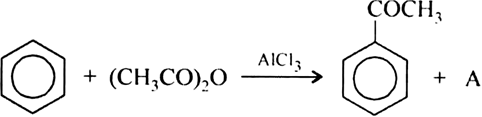55.
What is the correct order of increasing boiling point?
I: CH3CH2CH2OH,
II:CH3CH2OCH3,
III:CH3CH2CHO,
IV:CH3CH2CH3.
Propanol are associated due to extensive intermolecular hydrogen bonding, therefore, the boiling point of butan-1-ol would be the highest. Butanal is more polar than ethoxymethane. Therefore, the intermolecular dipole- dipole attraction is stronger in the former. n- propane molecule is have only weak van der waals forces. Hence increasing order of boiling point of the given compounds is as follows:
CH3CH2CH3 < CH3CH2OCH3 < CH3CH2CHO < CH3CH2CH2OH
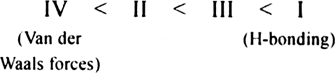
154 Views
 Short Answer Type
Short Answer Type