Short Answer Type
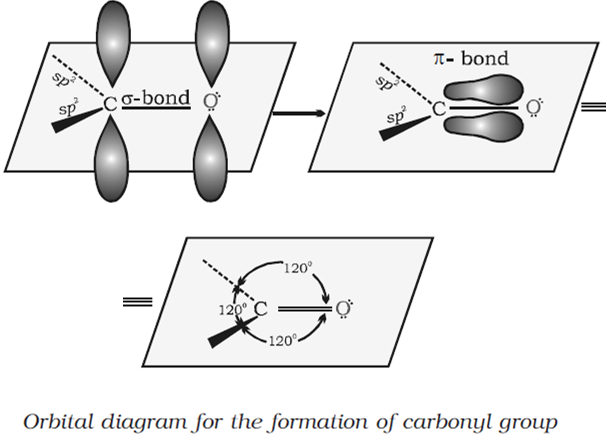
Short Answer TypeThe carbonyl carbon atom is sp2 -hybridised and forms three sigma bond. The fourth valence electron of carbon remains in its p-orbital and forms pi-bond with oxygen by overlapping with p- orbital of an oxygen. Also, the oxygen atom also has two non bonding electron pairs. Thus the carbonyl carbon and the three atoms attached to it lie in the same plane and the pi-electron is above and below this plane. The bond angle are approximately 1200.