 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWrite structural formulae and names of the four possible aldol condensation products from propanal and butanal. In each case, indicate which aldehyde served as nucleophile and which as electrophile.
Case i) Aldol condensation in two molecules of butanal, in which one acts as a nucleophile and the other as an electrophile.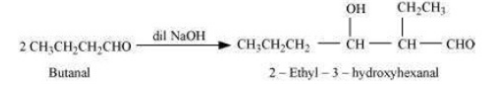
Case ii) Aldol condensation in two molecules of propanal, in which one acts as nucleophile and other as an electrophile.
Case iii) Aldol condensation in between of one molecule of propanal and butanal in which propanal acts as a nucleophile and butanal acts as an electrophile.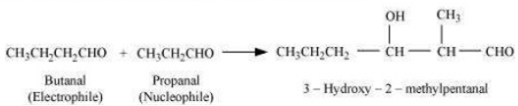
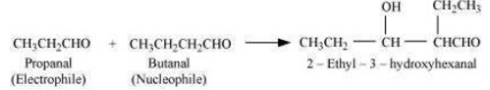
 Short Answer Type
Short Answer TypeAn organic compound (A) (Mol. formula C8H16O2) was hydrolysed with dilute sulphuric acid to give a carboxylic acid (B) and an alcohol (C). Oxidation of (C) with chromic acid produced (B). Write equations for the reactions involved.
