Short Answer Type
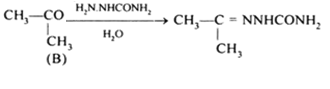
Short Answer TypeOrganic compound A is an alcohol as it gives a reaction with Na metal and contains one oxygen.
B is either an aldehyde or ketone but as it gives yellow ppt. with I2/NaOH, it is a ketone, CH3COCH3. Therefore A is a secondary alcohol.