 Short Answer Type
Short Answer TypeAccount for the following:
Carboxylic acids with five or less carbons are water soluble, but many with six or more carbons dissolves in alcohols.
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeEquation for the ionization of RCOOH is,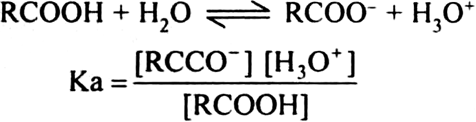
RCOOH is a stronger acid and has a lower pKa value than alcohol because RCOO- is more resonance stabilized than RO- ion.![]()
