 Short Answer Type
Short Answer TypeExplain the reason why:
Carbon-oxygen bond lengths in formic acid are 1.23 Å and 1.36 Å, but both the carbon-oxygen bonds in sodium formate have the same value, 1.27 Å.
Explain the reason why:
Acetic acid can be halogenated in the presence of red P and Cl2 but formic acid cannot be halogenated in the same way.
Explain the reason why:
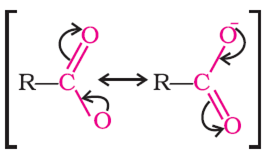
The carboxylate ion, RCOO- is more stable than the carboxylic acid, RCOOH.
Explain the reason why:
Carboxylic acids, despite the presence of C = O group in the molecule, do not form oximes, hydrazones, etc.
 Fill In the Blanks
Fill In the BlanksWhen a mixture of acetic acid and formic acid vapour is passed over MnO catalyst at 573 K, _________ is formed.
