 Short Answer Type
Short Answer TypeDo the following conversions in not more than two steps :
Ethyl benzene to Benzoic acid
pKa value of 4-nitrobenzoic acid is lower than that of benzoic acid.
It is due to e– with drawing nature of –NO2 attach at the para position of Benzene due to which tends to lose H+ ion increases and acidic character increases.
(A), (B) and (C) are three non-cyclic functional isomers of a carbonyl compound with molecular formula C4H8O .
Isomers (A) and (C) give positive Tollen’s test whereas isomer (B) does not give Tollen’s test but gives positive Iodoform test. Isomers (A) and (B) on reduction with Zn(Hg)/conc. HCl give the same product (D).
(a) Write the structures of (A), (B), (C) and (D)
(b) Out of (A), (B) and (C) isomers, which one is least reactive towards addition of HCN ?
Write the structure of the alkene formed by dehydrohalogenation of 1-bromo-1-methylcyclohexane with alcoholic KOH.
 Multiple Choice Questions
Multiple Choice QuestionsWrite IUPAC name of following compound
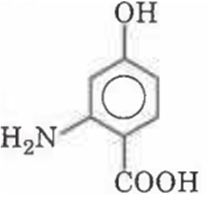
2-amino-4-hydroxybenzoic acid
6-amino-4-hydroxybenzoic acid
3-amino-4-carboxyphenol
2-carboxy-4-hydroxyaniline
