 Multiple Choice Questions
Multiple Choice QuestionsIdentify the wrong statement.
Salicylic acid is a monobasic acid
Methyl salicylate is an ester
Salicylic acid gives violet colour with neutral ferric chloride as well as brisk effervescence with sodium bicarbonate
Methyl salicylate does not occur in mineral oils
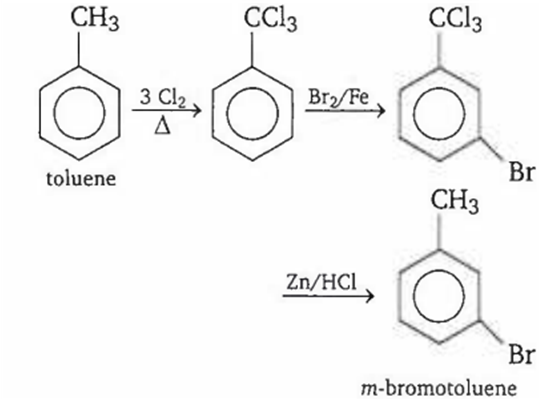
The compound 'C' in the following reaction is
o-bromotoluene
m-bromotoluene
p-bromotoluene
3-bromo-2, 4, 6-trichlorotoluene
B.
m-bromotoluene
Key Idea: Side chain hydrogen atoms are substituted in presence of light or heat. Ring hydrogens are substituted in presence of Lewis acid.
Benzaldehyde on refluxing with aqueous alcoholic KCN produce
cyanobenzene
cyanohydrin
benzoyl cyanide
benzoin
Reaction of acids with alcohols is also known as
esterification
saponification
alkalisation
None of these
