 Multiple Choice Questions
Multiple Choice QuestionsWhich one of the following reagents can distinguish between an aldehyde and a ketone?
Fehling's solution
H2SO4 solution
NaHSO4
NH3
Dry distillation of the mixture of calcium formate and calcium acetate gives
acetone
acetaldehyde
formaldehyde
formic acid
Trioxane has the formula ![]() , it is prepared from
, it is prepared from
formaldehyde
methanol
dichloromethane
vinyl alcohol
When methyl cyanide is hydrolysed in presence of alkali, the product is
acetamide
methane
CO2 + H2O
acetic acid
Which of the following reacts with phenol to give salicylaldehyde after hydrolysis?
Dichloro methane
Trichloro methane
Methyl chloride
None of these
B.
Trichloro methane
Phenol reacts with chloroform (trichloromethane) in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product and gives salicyladehyde. This reaction is called Reimer-Tiemann reaction.
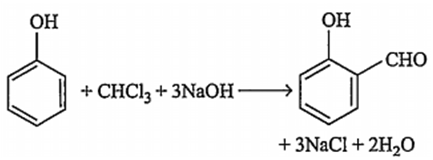
Note : In this reaction dichlorocarbene intermediate is formed.
