 Multiple Choice Questions
Multiple Choice QuestionsThe reaction CH3CH2ONa + BrCH2CH3 CH3CH2OCH2CH3 is called
Frankland reaction
Wurtz reaction
Williamson's synthesis
Cannizaro's reaction
Schiff's reagent is
p-rosaniline hydrochloride solution decolourised with sulphurous acid
magenta solution decolourised with Cl2
ammoniacal silver nitrate solution
alkaline KMnO4 solution
In which of the following compounds carboxylic group (-COOH) is not present?
Acetic acid
Lactic acid
Benzoic acid
Picric acid
When calcium acetate mixed with calcium formate is distilled, which of the following is not obtained?
Acetone
Formaldehyde
Acetaldehyde
Propionaldehyde
Glucose response to silver mirror test due to the presence of
-COOH group
an alkaline group
a ketonic group
an aldehydic group
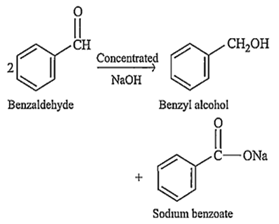
The reaction of benzaldehyde with alkali gives
phenol+ sodium benzoate
benzene + benzyl alcohol
benzyl alcohol + sodium benzoate
phenol+ benzene
C.
benzyl alcohol + sodium benzoate
Aldehydes which do not contain an α-hydrogen atom, treated with concentrated alkali solution to give alcohol and salt of carboxylic acid is called Cannizaro reaction. Example - Benzaldehyde reacts with alkali (NaOH) to give benzyl alcohol and sodium benzoate.
Reduction of an aldehyde gives
primary alcohol
monocarboxylic acid
secondary alcohol
tertiary alcohol
Which of the following is strongest base?
Perkin's reaction
Knoevenagel reaction
Reformatsky reaction
Benzoin condensation
