 Multiple Choice Questions
Multiple Choice QuestionsThe reaction of an organic compound with ammonia followed by nitration of the product gives a powerful explosive, called RDX. The organic compound is
phenol
toluene
glycerine
formaldehyde
The reagent which does not give acid chloride on treating with a carboxylic acid is
PCl5
Cl2
SOCl2
PCl3
Identify the organic compound which, on heating with strong solution of NaOH, partly converted into an acid salt and partly into alcohol
benzyl alcohol
acetaldehyde
acetone
benzaldehyde
When a mixture of methane and oxygen is passed through heated molybdenum oxide, the main product formed is
methanoic acid
ethanal
methanol
methanal
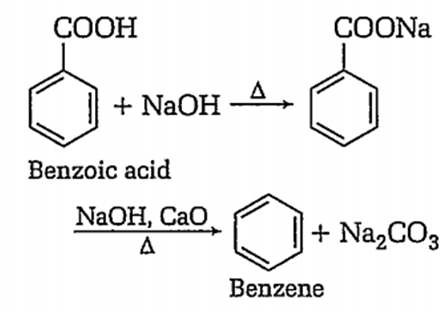
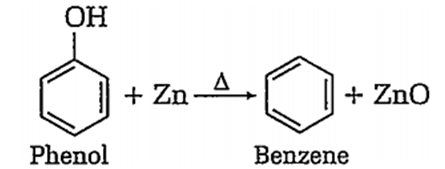
Benzene can be obtained by heating either benzoic acid with X or phenol with Y. X and Y respectively are,
zinc dust and soda lime
soda lime and zinc dust
zinc dust and sodium hydroxide
soda lime and copper
B.
soda lime and zinc dust
Benzene can be obtained by heating benzoic acid with soda-lime.
Benzene can also be obtained by heating phenol with zinc-dust.
An organic compound is boiled with alcoholic potash. The product is cooled and acidified with HCl. A white solid separates out. The starting compound may be
ethyl benzoate
ethyl formate
ethyl acetate
methyl acetate
An important reaction of acetone is autocondensation in presence of concentrated sulphuric acid to give the aromatic compound
mesitylene
mesityl oxide
trioxan
phorone
A nitrogen containing organic compound gave an oily liquid on heating with bromine and potassium hydroxide solution. On shaking the product with acetic anhydride, an antipyretic drug was obtained. The reactions indicate that the starting compound is
aniline
benzamide
acetamide
nitrobenzene
Benzyl alcohol and sodium benzoate is obtained by the action of sodium hydroxide on benzaldehyde. This reaction is known as
Perkin's reaction
Cannizaro's reaction
Sandmeyer's reaction
Claisen condensation
A compound, containing only carbon, hydrogen and oxygen, has a molecular weight of 44. On complete oxidation it is converted into a compound of molecular weight 60. The original compound is
an aldehyde
an acid
an alcohol
an ether
