 Multiple Choice Questions
Multiple Choice QuestionsWhich of the following gives an aldehyde on dry distillation?
Calcium formate + calcium acetate
Calcium acetate + calcium benzoate
Calcmm acetate
Calcium benzoate
The IUPAC name of ![]()
2-methyl-3-bromohexanal
3-bromo-2-methylbutanal
2-methyl-3-bromobutanal
3-bromo-2-methylpentanal
An organic compound 'A' burns with a sooty flame. It is negative towards Tollen's reagent test and positive for Borsche's reagent test. The compound 'A' is
acetophenone
acetone
salicylic acid
benzaldehyde
IUPAC name of H3C—(OH)CH—CH2—CHCOOH—CH3 is
4-hydroxy-1-methyl pentanoic acid
4-hydroxy-2-methyl pentanoic acid
2-hydroxy-4-methyl pentanoic acid
2-hydroxy-2-methyl pentanoic acid
The relative acidic strengths of benzoic acid, o-toluic acid and p-toluic acid is of the decreasing order
o-toluic acid > p-toluic acid > benzoic acid
p-toluic acid > benzoic acid > o-toluic acid
o-toluic acid > benzoic acid > p-toluic acid
p-toluic acid > o-toluic acid > benzoic acid
Conversion of benzene to acetophenone can be brought by
wurtz reaction
wurtz- fittig's reaction
Fridel crafts alkylation
Friedel crafts acylation
The compound formed when calcium acetate and calcium formate is distilled
acetone
acetaldehyde
benzaldehyde
acetophenone
Acetone and propanal are
functional isomers
position isomers
geometrical isomers
optical isomers
The reagent used to distinguish between acetaldehyde and benzaldehyde is
Tollen's reagent
Fehling's solution
2-4-dinitrophenyl hydrazine
semicarbazide
B.
Fehling's solution
(i) Reaction with 2,4-dinitrophenylhydrazine ( Brady's reagent).Acetaldehyde and benzaldehyde react wth 2,4-dintrophenylhydrazine (DNP) to form yellow, orange or red ppt of 2,4-dinitrophenylhydrazones (DNP derivatives).

(ii) Reaction with semicarbazide. Both acetaldehyde and benzaldehyde react with semicarbazide to form.
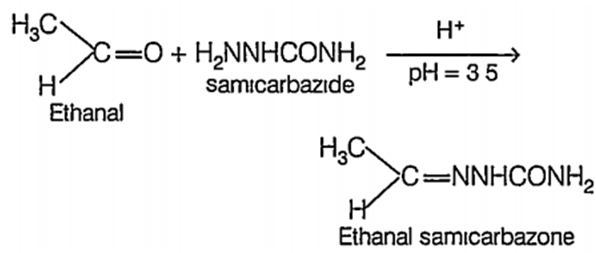
(iii) Reduction of tollen's reagent both acetaldehyde and benzaldehyde reduce.Tollen's reagent to metallic silver which deposit on the walls of the test tube as bnght silver mirror.
(iv)Reduction of Fehling's solution : Acetaldehyde reduces Fehling solution to a red ppt of cuprous oxide.
However, benzaldehyde does not reduce Fehling's solution.
