 Multiple Choice Questions
Multiple Choice QuestionsThe IUPAC name of the following compound is:
H3C--CH2-COOH
3-Hydroxy-4-methylpentanoic acid
4,4-Dimethyl-3-hydroxybutanoic acid
4-Methyl-3-hydroxypentanoic acid
2-Methyl-3-hydroxypentan-5-oic acid
In the following reaction carbonyl compound +MeOH acetal.
Rate of the reaction is highest for:
Acetone as substrate and methanol in excess
Propanal as substrate and methanol in stoichiometric amount.
Propanal as substate and methanol in excess.
Acetone as substrate and methanol in stoichiometric amount.
The major product of the following reaction is:
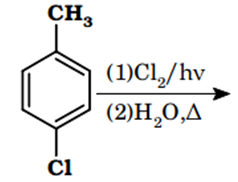
![]()
![]()
![]()
![]()
C.
![]()
Compound A(C9H10O) shows positive iodoform rest. Oxidation of A with KMnO4/ KOH gives B(C8H6O4). Anhydride of B is used for the preparation of phenolpthalein. Compound A is:
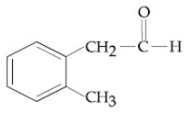
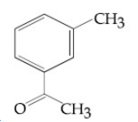
![]()
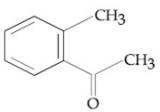
Which one will not show Cannizzaro reaction ?
Trimethyl acetaldehyde
Formaldehyde
Acetaldehyde
Benzaldehyde
The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha -carbon, is
a carbonyl compound with a hydrogen atom on its alpha -carbon rapidly equilibrates with its corresponding enol and this process is known as aldehyde-ketone equilibration.
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as carbonylation
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism
a carbonyl compound with a hydrogen atom on its alpha-carbon rapidly equilibrates with its corresponding enol and this process is known as keto-enol tautomerism
