 Multiple Choice Questions
Multiple Choice QuestionsIn a set of reactions, acetic acid yielded a product D.
CH3COOH A B C D
The structure of D would be
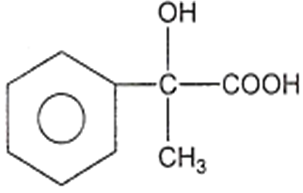
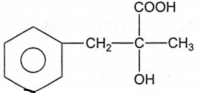
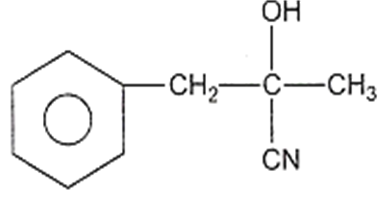
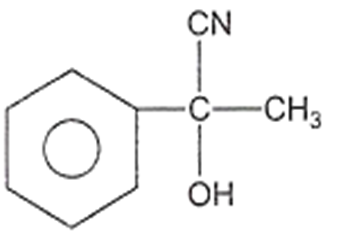
Aldehydes or ketones are treated with-bromo ester in the presence of zinc to form -hydroxy ester followed by dehydration to form ,-unsaturated ester.This reaction is known as:
Witting reaction.
Tischenko reaction.
Reformatsky reaction.
Crossed-cannazzaro's reaction.
The correct structure of the stable compound which is obtained when acetophenone react with a base C2H5OH is
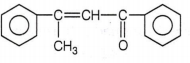
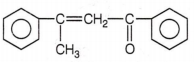
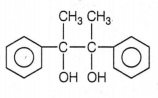
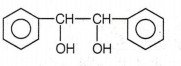
CH3CH2CCH butanone-2. R is
Hg++/ dil.H2SO4
KMnO4
KClO3
K2Cr2O7
A.
Hg++/ dil.H2SO4
In the above given reaction, R is Hg++/ dilute. H2SO4
This is because alkyne changes to ketone upon hydrolysis in presence of mercury salt.
CH3CH2CCH CH3CH2COCH3 (butanone-2)
