 Multiple Choice Questions
Multiple Choice QuestionsThe Kolbe's electrolysis proceeds via
nucleophilic substitution mechanism
electrophilic addition mechanism
free radical mechanism
electrophilic substitution reaction
The reagent used in Gatterman Koch aldehyde synthesis is :-
Pb/ BaSO4
alkaline KMnO4
acidic KMnO4
CO + HCl
Iodoform can be obtained on warming NaOH and iodine with
CH3-CH2-CH(OH)CH3
(CH3)2CH-CO-C2H5
CH3-CO-OCH3
(CH3)3CCH2OH
In Williamson's synthesis
an alkyl halide is treated with sodium alkoxide
an alkyl halide is treated with sodium
an alcohol is heated with cone. H2SO4 at 130C
None of the above
The Cannizaro reaction is not given by
trimethyl acetaldehyde
acetaldehyde
benzaldehyde
formaldehyde
Self condensation of acetaldehyde, in the presence of dilute alkalies gives
an acetal
an aldol
mesitylene
propionaldehyde
B.
an aldol
The self condensation of acetaldehyde in presence of dilute alkalies is called aldol condensation.
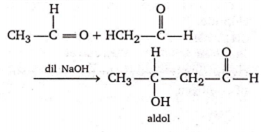
Stephen's reduction is used to prepare aldehyde from
alcohol
alkyl cyanides
alkanones
acid chlorides
