 Long Answer Type
Long Answer Type Short Answer Type
Short Answer TypeWith the help of inductive effect, show that monochloroacetic acid is stronger acid than acetic acid.
Which bond is more polar in the following pairs of molecules:
(i) H3C – H, H3C – Br
(ii) H3C – NH2, H3C – OH
(iii) H3C – OH, H3C – SH
 Long Answer Type
Long Answer TypeExplain the terms Inductive and Electromeric effects. Which electron displacement effect explains the following correct orders of acidity of the carboxylic acids?
Or
How will you explain the following correct orders of acidity of the carboxylic acids?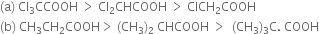
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer Type