 Long Answer Type
Long Answer TypeWrite resonance structures of CH2 = CH–CHO, indicate relative stability of the contributing structures.
 Short Answer Type
Short Answer TypeGive reasons why the following two structures I and II cannot be the major contributors to the real structure of CH3COOCH3: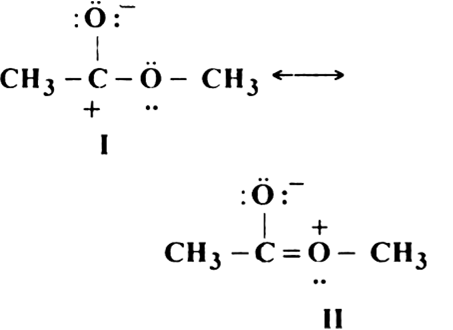
 Long Answer Type
Long Answer Type Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are carbocations? Discuss the relative stabilities of primary, secondary and tertiary carbocations.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are carbanions? How are these generated? Discuss the relative stabilities of primary, secondary and tertiary carbanions.
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeWhat are free radicals? How are these formed? Discuss the structure of free radicals.

