 Multiple Choice Questions
Multiple Choice QuestionsOf the five isomeric hexanes, the isomer which can give two monochlorinated compounds is
n-hexane
2, 3-dimethylbutane
2,2-dimethylbutane
2,2-dimethylbutane
An organic compound having molecular mass 60 is found to contain C = 20%, H = 6.67% and N = 46.67% while rest is oxygen. On heating, it gives NH3 along with a solid residue. The solid residue give violet colour with alkaline copper sulphate solution. The compound is
CH3NCO
CH3CONH2
(NH2)2CO
(NH2)2CO
The ammonia evolved from the treatment of 0.30 g of an organic compound for the estimation of nitrogen was passed in 100 mL of 0.1 M sulphuric acid. The excess of acid required 20 mL of 0.5 M sodium hydroxide solution hydroxide solutio for complete neutralization. The organic compound is
acetamide
thiourea
urea
urea
When metal ‘M’ is treated with NaOH, a white gelatinous precipitate ‘X’ is obtained, which is soluble in excess of NaOH. Compound ‘X’ when heated strongly gives an oxide which is used in chromatography as an adsorbent. The metal ‘M’ is
Fe
Zn
Ca
Al
Which statement is not correct for ortho and para hydrogen?
They have different boiling points
Ortho-form is more stable than para-form
They differ in their nuclear spin
The ratio of ortho to para hydrogen changes with change in temperature
In the IUPAC system, PhCH2CH2CO2H is named as
3-phenylpropanoic acid
benzylacetic acid
carboxyethylbenzene
2-phenylpropanoic acid
The correct order of acid strengths of benzoic acid (X), peroxybenzoic acid (Y) and p-nitrobenzoic acid (Z) is
Y > Z > X
Z > Y > X
Z > X > Y
Y > X > Z
Choose the correct statement(s) among the following.
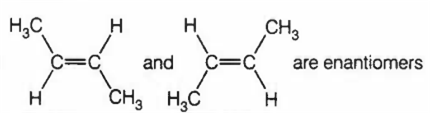
CH3CHO on reaction with HCN gives racemic mixture

CH3-CH=NOH shows geometrical isomerism
Optical isomerism is exhibited by (ox= oxalate anion; en= ethylenediamine).
cis-[CrCl2(ox)2]3-
[Co(en)3]3+
trans-[CrCl2(ox)2]3-
[Co(ox)(en)2]+
