 Multiple Choice Questions
Multiple Choice QuestionsAmong the following statements about the molecules X and Y, the one(s) which correct is (are)
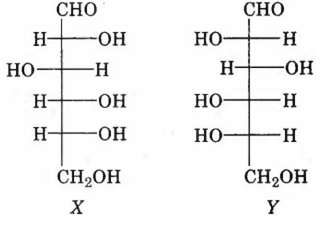
X and Y are diastereomers
X and Y are enantiomers
X and Y are both aldohexoses
Xis a D-sugar and Y is an L-sugar
Amongst the following, the one which can exist in free state as stable compound is
C7H9O
C8H12O
C6H12O
C10H17O
 Short Answer Type
Short Answer TypeCompound A treated with NaNH2 followed by CH3CH2Br gave compound B. Partial hydrogenation of compound B produced compound C, which on ozonolysis gave a carbonyl compound D, (C3H6O). Compound D did not respond to iodoform test with I2/KI and NaOH. Find out the structures of A, B, C and D.
An organic compound with molecular formula C9H10O forms 2, 4-DNP derivative, reduces Tollen's reagent and undergoes Cannizaro reaction. On vigorous oxidation it gives a dicarboxylic acid which is used in the preparation of terylene. Identify the organic compound.
 Multiple Choice Questions
Multiple Choice QuestionsThe well known compounds, ( +) - lactic acid and (-) - lactic acid, have the same molecular formula, C3H6O3. The correct relationship between them is
constitutional isomerism
geometrical isomerism
identicalness
optical isomerism
The stability of Me2C=CH2 is more than that of MeCH2CH=CH2 due to
inductive effect of the Me groups
resonance effect of the Me groups
hyperconjugative effect of the Me groups
resonance as well as inductive effect of the Me groups
