 Multiple Choice Questions
Multiple Choice QuestionsIn Dumas method, 0.3 g of an organic compound gave 45 mL of nitrogen at STP. The percentage of nitrogen is
16.9
18.7
23.2
29.6
The IUPAC name of the following compound is-
(CH3)2CH-CH=CH-CH=CH(C2H5)-CH3
2, 7-dimethyl-3, 5-nonadiene
2, 7-dimethyl-2-ethylheptadene
2-methyl-7-ethyl-3, 5-octadiene
1, 1-dimethyl-6-ethyl-2, 4-heptadiene
Identify the correct statements from the following
(i) Electromeric effect is a permanent effect
(ii) Hyper conjugation is a temporary effect
(iii) Fractional distillation is used to separate two liquids from a mixture if the difference in their boiling points is less
(iv) Different compounds are adsorbed on an adsorbent different extents
ii, iii, iv
i, ii, iii
ii, iv
iii, iv
Ferric ion forms a Prussian blue coloured precipitate due to formation of
K4[Fe(CN)6]
Fe4[Fe(CN)6]3
Fe(CNS)3
K3[Fe(CN)6]
Which of the following will show geometrical isomerism?
1-butene
2-butene
2-methyl propene
Propene
n-propyl alcohol and iso-propyl alcohol are examples of
position isomerism
chain isomerism
tautomerism
geometrical isomerism
The IUPAC name of the following compound is:
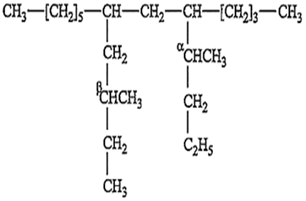
7 [β-methyl butyl]; 9 butyl tridecane
3 [β-ethyl butyl]; 9 ethyl tridecane
2 [β-ethyl ethenyl]; 8 propyl decane
None of the above
IUPAC name of [Pt(NH3)2Cl2] is
diamine dichloro platinum (II)
amine, chloro platinum (III)
chloro diamine platinum (II)
None of the above
