 Multiple Choice Questions
Multiple Choice QuestionsWhich of the statements is not true?
On passing H2S through acidified K2Cr2O7 solution, milky colour is observed.
Na2Cr2O7 is preferred over K2Cr2O7 in volumetric analysis
Na2Cr2O7 solution in acidic medium is orange
K2Cr2O7 solution becomes yellow on increasing the pH beyond 7
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear.
CH3 - C ≡ C - CH3
CH2 = CH - CH2 - C ≡ CH
CH3 - CH2 - CH2 - CH3
CH3 - CH2 - CH2 - CH3
The Lassaigne's extract is boiled with conc HNO3 while testing for halogens. By doing so it
helps in the precipitation of AgCl
increases the solubility product of AgCl
increases the concentration of NO3- ions
increases the concentration of NO3- ions
The correct IUPAC name of the compound of 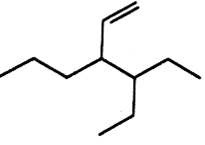
3 - ethyl - 4 ethenyl heptane
3 - ethyl - 4 - propylhex- 5-ene
3-(1-ethyl propyl) hex - 1 ene
3-(1-ethyl propyl) hex - 1 ene
Which of the following statement is correct for a nucleophile?
Nucleophile is s Lewis acid
Ammonia is a nucleophile
Nucleophiles attack low electrons density sites
Nucleophiles attack low electrons density sites
The number of structural isomers possible from the molecular formula C3H9N is
4
5
2
2
