 Multiple Choice Questions
Multiple Choice Questions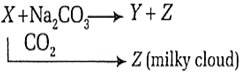
The chemical formulae of X, Y and Z are
| X | Y | Z |
| CaO | Ca(OH)2 | NaOH |
| NaOH | CaO | CaCO3 |
| NaOH | Ca(OH)2 | CaCO3 |
| Ca(OH)2 | NaOH | CaCO3 |
What is correct about the following structure?
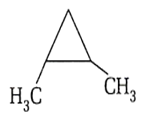
Total stereoisomers = 4
Number of chiral carbons = 1
Number of optical isomers= 2
Number of meso compounds = 2
During estimation ofnitrogen in the organic compound by Kjeldahl's method, the ammonia evolved from 0.5 g of the compound in Kjeldahl's estimation of nitrogen, neutralised 10 mL of 1M H2SO4. Find out the percentage of nitrogen in the compound.
14%
28%
56%
68%
On addition of conc. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet fumes come out. This is because
H2SO4 reduces HI to I2
HI is of violet colour
HI gets oxidised to I2
HI changes to HIO3
Affinity for hydrogen decreases in the group from fluorine to iodine. Which ofthe halogen acids should have highest bond dissociation enthalpy?
HF
HCl
HBr
HI
Acetic anhydride is prepared in the laboratory by heating sodium acetate with
ethyl chloride
acetyl chloride
conc. H2SO4
zinc dust
The structures of (CH3)3CBr and CH3[CH2]3 Br represent
chain isomerism
position isomerism
chain as well as position isomerism
functional isomerism
Petrol for aviation purpose must contain
straight chain hydrocarbons
aromatic hydrocabrons
olefinic hydrocarbons
highly branched chain paraffins
