 Long Answer Type
Long Answer TypeIn a Duma's nitrogen estimation method, 0.3 g of an organic compound gave 50 mL of nitrogen collected at 300K and 715 mm pressure. Calculate the percentage of nitrogen in the compound. (Aqueous tension of water at 300 K is 15 mm).
 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeDiscuss a suitable method for the estimation of halogens.
Carius method for the estimation of halogens: A known mass of the organic compound containing halogens is heated with an excess of fuming nitric acid and silver nitrate in a sealed tube called Carius tube. Cabron, hydrogen and sulphur (if present) are oxidised to CO2, H2O and H2SO4 respectively whereas halogen forms a precipitate of silver halide. The precipitate is separated, washed with distilled water, dried and weighed. The percentage of halogens is then calculated. Calculations:
Let the mass of the compound taken = Wg Let the mass of halide (AgX) formed = ag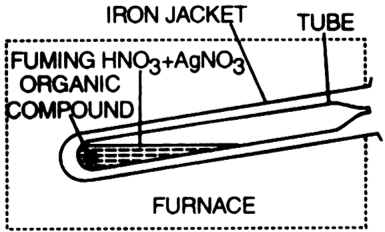
Atomic mass of halogen = x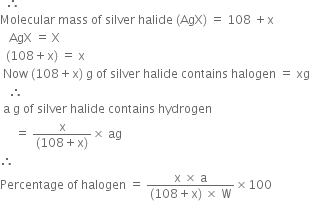
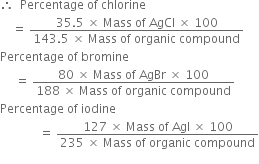
 Short Answer Type
Short Answer TypeIn Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. Find out the percentage of bromine in the compound.
0.15 g of iodoform gave 0.2682 g of silver iodide. Calculate the percentage of iodine.
