 Short Answer Type
Short Answer Type Long Answer Type
Long Answer TypeGive reasons for the following:
(a) PCl5 acts as a chlorinating agent in organic reactions.
(b) Nitric oxide becomes brown when released in air.
(c) PCl5 is ionic in nature in the solid state.
(d) Ammonia acts as a ligand.
(e) Sulphur disappears when boiled with an aqueous solution of sodium sulphite.
A translucent waxy solid (A) on heating in an inert atmosphere is converted to its allotropic from (B). Allotrope (A) on reaction with very dilute aqueous KOH liberates highly poisonous gas (C) having rotten fish smell. With excess of chlorine forms (D) which hydrolyses to compound (E).
Identify compound (A) to (E)
Or
Concentrated sulphuric acid is added followed by heating to each of the following test tube labelled (i) to (v).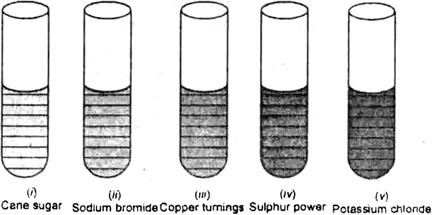
Identify in which of the above test tube the following change will be observed. Support your answer with the help of a chemical equation.
(a) Formation of black substance
(b) Evolution of brown gas
(c) Evolution of colourless gas
(d) Formation of brown substance which on dilution becomes blue.
(e) Disapperance of yellow powder along with evolution of colourless gas.
 Short Answer Type
Short Answer Type