 Short Answer Type
Short Answer TypeThe three equatorial P–Cl bonds are equivalent, while the two axial bonds are longer than equatorial bonds. This is due to the fact that the axial bond pairs suffer more repulsion as compared to equatorial bond pairs.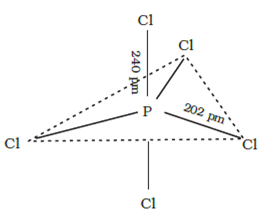
 Fill In the Blanks
Fill In the Blanks